ED ACS Adult Guideline revision 2020
- Guideline
- Diagnosis and Management of Acute Coronary Syndrome (Adult)
- Revision Date
- 2020
This is a sub-page of the page titled ACS (Acute Coronary Syndome) in the Emergency Department.
Target population
ACS (Acute Coronary Syndrome) is the term used to describe myocardial ischemia which results from the acute occlusion (either partial or complete) of a coronary artery.
This guideline is intended to expedite the diagnosis and management of ED patients presenting with signs/symptoms suggestive ACS. In this context, “suggestive” should be interpreted to mean that ACS is the most likely etiology; or, from a different perspective, the clinician feels that the probability of ACS is high enough that the condition is “ACS until proven otherwise.” Such patients are only a subset of those who present complaining of “chest pain” and thus this guideline is not intended to inform the management of all patients complaining of chest pain.
Type-2 cardiac ischemia (i.e. cardiac oxygen supply-demand imbalance not due to acute coronary occlusion) is not ACS. This guideline is not intended to guide the diagnosis and/or management of type-2 cardiac ischemia.
Impetus for guideline revision
- Last update was in 2011, and several interim events have occurred:
- The Third Universal Definition of Myocardial Infarction was published in 2012[1]; this changed the EKG criteria for diagnosis of STEMI.
- For purposes of immediate management, 2014[2] and 2015[3] international ACS guidelines combined NSTEMI and unstable angina into the composite NSTE-ACS (Non-ST-Elevation ACS).
- The Fourth Universal Definition of Myocardial Infarction was published in 2018[4]; this introduced the terms "acute myocardial injury" and "chronic myocardial injury".
- On November 12, 2019, the Yukon-Kuskokwim Delta Regional Hospital (YKDHR) switched cardiac troponin assay to the Roche Diagnostics Elecsys® Troponin T Gen 5 STAT. Details are available in the manufacturer's package insert.
Goals
- The guideline should be quickly useful without having previously studied it.
- All necessary medications and doses should be included in the guideline.
- Major cautions and contraindications should be included in the guideline.
- Clinicians with basic ED and/or Urgent Care skills who rarely diagnose and treat ACS should be able to use this guideline to correctly diagnose and manage most straightforward ACS cases (i.e. the majority of them) without external information sources. Unfortunately, some cases are not straightforward and can be challenging for even the most experienced experts; such cases are frequently not amenable to guideline-based management and early expert consultation may be warranted in order to individualize care.
Changes
Major
- This is a ground-up rewrite without significant inheritance.
- Utilization of high-sensitivity troponin-T test.
- New STEMI diagnostic criteria from the Third Universal Definition of Myocardial Infarction (2012).[1] These EKG criteria are unchanged in the Fourth Universal Definition of Myocardial Infarction (2018).[4] (Left bundle-branch block removed, lead- and age-specific elevation measurements included)
- Use of the new term NSTE-ACS (Non-ST-Elevation Acute Coronary Syndrome; includes the two entities known as NSTEMI and unstable angina)
- Oxygen is no longer mandatory, but rather titrated to achieve normoxemia. Oxygen should NOT be administered unless the patient is hypoxic (SpO2 < 90%), and supplemental O2 should NOT push the SpO2 above 96%.
- Enoxaparin dosing (in STEMI) is now adjusted for age and renal function.
- Morphine is no longer recommended (though neither is it recommended against).
- Fibrinolytic contraindications are on the fibrinolytic checklist and are not duplicated on the guideline.
- Inclusion of DAPT (Dual Anti-Platelet Therapy, which refers specifically to combining aspirin with a P2G12 inhibitor).
- Switch from alteplase to tenecteplase as the thrombolytic agent for STEMI reperfusion.
Minor
- For STEMI with age<75y, enoxaparin is started with an IV Bolus.
Rationale for Specific Recommendations
- EKG prior to Immediate Interventions
-
- YKDRH has limited inpatient capabilities in this context (no cardiologist, no telemetry). Dynamic ST/T changes are diagnostic for unstable angina and therefore an indication for MedEvac transfer to a higher level of care. Lack of EKG done while in pain (i.e. prior to NTG) can substantially delay diagnosis of unstable angina.
- Enoxaparin for anticoagulation
-
- - Time to angiography will be at least 6 hours and possibly up to 12-18 hours.
- - Our ED has limited resources, and in this setting more complexity increases the likelihood of errors.
- - Care will transition to a MedEvac crew; again, more complexity increases likelihood of errors.
- Enoxaparin IV Bolus for STEMI with age < 75y
-
- - recommended by the AHA/ACC 2013 STEMI guideline (see Secondary Information Sources below, pg e97, Table-1)
- - recommended by the latest UpToDate.com article (see Primary Information Sources below)
- - recommended by the South Central Alaska STEMI Committee's "Alaska Statewide STEMI Recommendations" (see Secondary Information Sources below)
- Switch from alteplase to tenecteplase
- Ease of administration
- Tenecteplase is a single weight-based bolus pushed over five seconds (compared to three different alteplase infusions over ninety minutes).
- The simplicity minimizes the risk of drug error.
- Rapidity of administration
- Standard-of-care is door-to-infusion time of <= 30 minutes.
- Given our resource limitations, we are very unlikely to achieve this standard with alteplase infusions.
- Efficiency
- An IV lumen is not exclusively occupied for 90 minutes.
- Nursing and MedEvac crews have more time to focus on other issues.
- Standard of care
- Per the UpToDate.com article Characteristics of fibrinolytic (thrombolytic) agents and clinical trials in acute ST elevation myocardial infarction, “Tenecteplase (TNK-tPA) is the fibrinolytic agent of choice within the United States, Canada, and many European countries.”
- Clopidrogel
- - 600mg loading dose for "STEMI > 12h"
- - the pharmacologic properties differentiating the different P2Y12 inhibitors are:
- Mechanism of action (direct versus indirect).
- Potency (with regards to both effectiveness and adverse effects)
- Onset time
- Recovery time
- Resistance
- - Recommendations from UpToDate reviews and national guidelines attempt to balance these effects for the different ACS scenarios.
- The "STEMI < 12h" category assumes the patient is receiving fibrinolytic therapy. In this setting, the risk of bleeding is much higher, so a relatively weak anti-platelet effect is desired.
- The two other ACS categories ("STEMI > 12h" and NSTE-ACS) will not receive fibrinolytics, therefore a much more potent anti-platelet effect is achievable without a substantial increased bleeding risk. This is why these patients receive a loading dose of a more potent agent (prasugrel or ticagrelor) or the very large loading dose of clopidrogel.
- Prasugrel has multiple caveats to using it (i.e. contraindications and/or cautions, such as Age > 75, wt < 60kg, any Hx prior TIA/stroke, etc.) which, during time-pressured treatment, could lead to it being used when it is more likely to cause harm (i.e. intra-cranial bleeding) than benefit .
- - One problem of clopidrogel in the ACS setting is its slow onset. This can be overcome with a loading dose, and 600mg gives fairly rapid onset without substantial bleeding risk.
- - We debated recommending clopidrogel at 300mg, 300-600mg, and 600mg, as well as the options of ticragrelor or prasugrel. In the end, the input from pharmacy (Jason Barret) settled the debate:
- This is a complicated question and there are multiple correct answers, so I will try and keep with the theme that we would like it to be simple. I will give my reasoning.
Just to echo Andy the dosing for clopidogrel following fibrinolytic therapy (STEMI <12 hours) is set (based on the PCI-CLARITY trial). For a patient who is not eligible for the fibrinolytic (STEMI >12 hours with the intent of primary PCI) based on the current (2013) guidelines for the management of STEMI loading doses of clopidogrel (600mg), Prasugrel (60mg) or Ticagrelor (180mg) are recommended (level of evidence: B). The most recent UpToDate “articles” that address the P2Y12 receptor blockers seems to lean more towards the Prasugrel or Ticagrelor: this is based on two studies the TRITON-TIMI and PLATO where they compared the two drugs to clopidogrel 300mg loading dose respectively. As mentioned below the benefit was small and there was more “minor” bleeding with the Prasugrel and Ticagrelor. Unfortunately the TRITON-TIMI trial and PLATO trial used clopidogrel 300mg as the LD. The CURRENT-OASIS 7 trial (2010) compares clopidogrel 300mg vs 600mg and found a benefit to the 600mg loading dose; major bleeding was more common with the larger loading dose (1.6% vs 1.1% p=0.009). Keeping with the “keeping it simple” theme I would recommend putting 600mg on the guideline because of the benefit and accessibility in the ED. Please note that based on the potential benefit and likely recommendations from outside sources for the Prasugrel and ticagrelor; it could be added to the pyxis, if needed (I would recommend not putting them in the pyxis, because the data is week at the moment, but could change in the future)…this can be up for discussion. That’s what I think, I hope this answered the question.
- This is a complicated question and there are multiple correct answers, so I will try and keep with the theme that we would like it to be simple. I will give my reasoning.
- - However, if a patient is deemed to be at a particularly high risk of bleeding, clopidrogel 300mg might me a better option; yet there is no evidence to support this supposition, so it is not in the guideline.
- - the pharmacologic properties differentiating the different P2Y12 inhibitors are:
- Recommendation to consult cardiology prior to giving clopidrogel in NSTE-ACS
-
- - This recommendation came from ANMC Cardiology.
- - Rationale: a single loading dose of clopidrogel requires waiting SEVEN days before a CABG can be performed. Cardiology requests being consulted about the particular details of a case prior to giving clopidrogel (or any P2Y12 inhibitor) in the setting of NSTE-ACS.
- Recommendation for intravenous (IV) beta-blocker for refractory angina
-
- - ANMC cardiology has recommended to use an oral, immediate-onset beta-blocker for this purpose.
- - However, YKDHR formulary only contains metoprolol XL. This formulation's slow onset is inappropriate for treatment of refractory angina during ACS.
- - Therefore, if a beta-blocker is necessary in this setting, it must be given IV.
- Not advocating urgent administration of a statin
- The SECURE-PCI trial (2018) directly tested early loading with a high-dose statin versus placebo and found no benefit on MACE (the primary outcome) or death (THE most patient-important outcome).[5][6]
- A Cochrane Review (2014) of this topic concluded no benefit.[7]
- At the time of this guideline revision, "TheNNT.com" also reports no benefit for acute statins during ACS.[8]
- Some prior trials showed a benefit to "in hospital" initiation of a high-dose statin (versus placebo), but these included patients started on a statin up to 10 days after the index event.[6]
- No trials have shown a harm of urgent high-dose statin administration during ACS. Therefore once the patient is stabilized and all the urgent medications are given, it is reasonable to administer a high-dose statin prior to transfer; but it should not be a priority and should not delay transfer.
Issues intentionally NOT addressed in this revision
- Diagnosis and management of “chest pain” which is not suggestive of acute myocardial ischemia.
- Diagnosis/Management of type-2 MI’s.
- HS-troponin diagnostic algorithms other than the FDA-approved cutoffs.
- Use of the terminology “acute cardiac injury” and “chronic cardiac injury”.
- Diagnosis/management of suspected ACS in a village clinic.
- Different f/u strategies based upon risk stratification (i.e. when ACS has been ruled-out).
Future Directions (i.e. for future revisions)
- Review/update this guideline every two years. Given the importance of the topic and the vast amount of active research, frequent updates are indicated.
- Review the status/results of the STREAM-2 trial,[9][10] which will provide direct evidence of fibrinolytic therapy benefit in the elderly population.
- Evidence-based troponin algorithm/cutoffs.
- - Based upon institutional data or published data?
- Evidence-based use of anticoagulation.
- A cochrane review of RCT's found that anticoagulation (i.e. heparins, both unfractionated and low-molecular-weight) for ACS yielded no effect on patient-important outcomes, a possible small decrease in subsequent nonfatal-MI, and increased bleeding.[11] Yet other respected evidence-based reviews have criticized that conclusion as optimistic and concluded harm without meaningful benefit.[12],[13],[14] However, anticoagulation persists in most guidelines, apparently due to an unhealthy combination of tradition and liability concerns. We should consider reviewing this topic and developing the clinical courage to remove heparin from the ACS guideline.
- Develop a similar guideline for suspected ACS in a village clinic.
- - It differs enough to warrant being a standalone guideline; incorporating it into the ED guideline risks making the ED guideline too unwieldy to be quickly usable.
- - This seems an ideal use of the HE-MACS score.
- Deep dive into who the relative contraindications for fibrinolytics are most appropriately applied to: only patients at low risk of cardiac death?
Primary Information Sources for Guideline Revision
ACS
- Barstow C, Rice M, McDivitt JD. Acute Coronary Syndrome: Diagnostic Evaluation. Am Fam Physician. 2017;95(3):170-177. (Archived)
Myocardial Infarction Definition
- Fourth Universal Definition of Myocardial Infarction, 2018 (Consensus statement by the ACC, ESC, AHA)[4]
STEMI
- UpToDate: Overview of the acute management of ST-elevation myocardial infarction (Current: Dec 2019, Updated: 01-28-2019)
- UpToDate: Antiplatelet agents in acute ST-elevation myocardial infarction (Current: Dec 2019, Updated: 09-17-2019)
- UpToDate: Anticoagulant therapy in acute ST-elevation myocardial infarction (Current: Dec 2019, Updated: 02-20-2019)
NSTE-ACS
- UpToDate: Overview of the acute management of non-ST elevation acute coronary syndromes (Current: Dec 2019, Updated: 12-13-2018)
- UpToDate: Antiplatelet agents in acute non-ST elevation acute coronary syndromes (Current: Dec 2019, Updated: 09-17-2018)
- UpToDate: Anticoagulant therapy in non-ST elevation acute coronary syndromes (Current: Dec 2019, Updated: 02-02-2019)
Secondary Information Sources for Guideline Revision
ACS
- Group GW and TN. Beta Blockers for Heart Attack. TheNNT. https://www.thennt.com/nnt/beta-blockers-for-heart-attack/. Accessed February 18, 2020. (Archived)
STEMI
- Group GW and TN. Thrombolytics for Major Heart Attack (STEMI). TheNNT. https://www.thennt.com/nnt/thrombolytics-for-major-heart-attack/. Accessed February 18, 2020. (Archived)
- Alaska Heart Institute: Alaska Statewide STEMI Recommendations (updated May 2017) (Archived)
- Alaska Heart Institute: Alaska Statewide STEMI Recommendations (Aug 2020)
NSTE-ACS
- 2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Archived)
- 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC) (Archived)
Additional Resources
High-sensitivity Troponin-T Interpretation
ECG Interpretation
Risk-Score Tools
Fibrinolytics
RV Infarct
- UpToDate: Right ventricular myocardial infarction
Benefit versus Harm data for fibrinolytic thereapy in STEMI
The generally quoted date for benefit from using fibrinolytics in STEMI is from the 1994 meta-analysis in the Lancet by the Fibrinolytic Therapy Trialists' (FTT-1994) group.[15] This study also reported harms, but two subsequent large population-based studies of harm are generally relied upon because history has shown that the rigid protocol adherence in RCT's results in an underestimate of true risk of harm experience in community practice. These two large population studies are Gurwitz-1998[16] and Brass-2000.[17] Of note, Gurwitz-1998 examined all age groups while Brass-2000 only examined Medicare patients (i.e. age >=65).
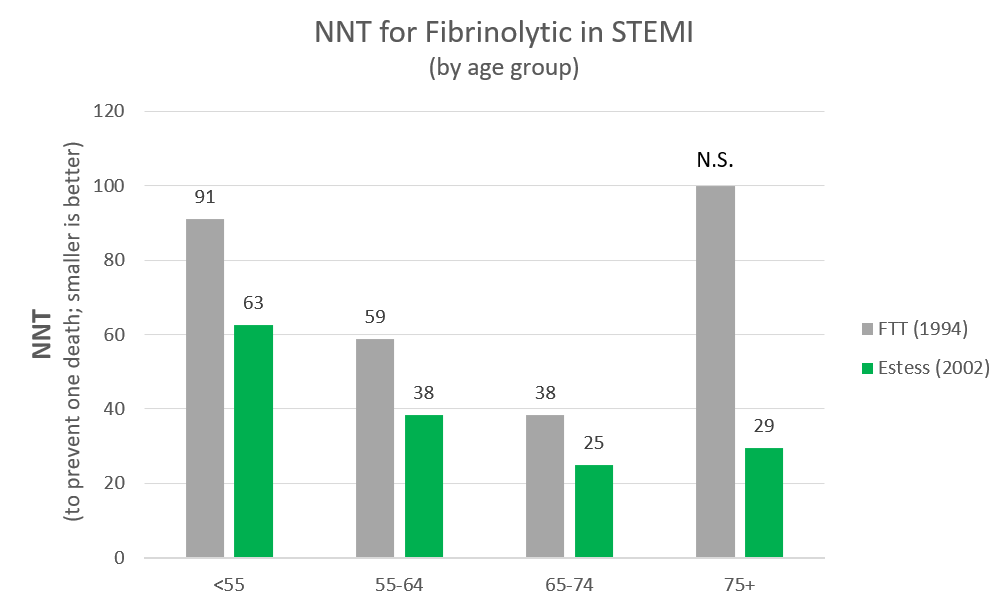
The mortality benefit by age group from FFT-1994 is summarized in the following table:
| Age | F | C | RR | ARR | NNT |
|---|---|---|---|---|---|
| <55 | 3.4% | 4.5% | 0.76 | 1.1% | 91 |
| 55-64 | 7.2% | 8.9% | 0.81 | 1.7% | 59 |
| 65-74 | 13.5% | 16.1% | 0.84 | 2.6 % | 38 |
| 75+ | 24.3% | 25.1% | 0.96 | 1% | 100 (not significant) |
The intracranial hemorrhage (ICH) risk data from all three of these studies (FTT, Gurwitz, and Brass) is summarized in the following table:
| All | <55 | 55-64 | <65 | 65-74 | 75+ | |
|---|---|---|---|---|---|---|
| FTT (1994)[15] | 1 in 257 | 1 in 1668 | 1 in 186 | - | 1 in 245 | 1 in 99 |
| Gurwitz (1998)[16] | - | - | - | 1 in 249 | 1 in 81 | 1 in 47 |
| Brass (2000)[17] | - | - | - | - | 1 in 88 | 1 in 53 |
This benefit/harm data contains a paradoxical/non-intuitive effect. Though the relative risk reduction is steady across the age groups and the risk of intracranial hemorrhage (ICH) increases with age, the NNT decreases with age (i.e. the treatment is more beneficial as age rises). This occurs because, as the age increases, the mortality from STEMI increases markedly. A similar relative risk reduction produces a much larger absolute risk reduction with a higher mortality rate, therefore the NNT is lower. But at age 75, the risk of ICH increases so dramatically that it overcomes the trend of increasing benefit with age and completely nullifies any benefit.
Understanding this data is critically important when considering likelihood of benefit versus harm. Age 65-74 appears to be the group which benefits most, but this age factor can combine with other relative contraindications to markedly increase the risk of ICH and thus nullify (or even reverse) the likelihood of benefit. Therefore age 65-74 is listed as a relative contraindication, and when combined with other relative contraindications (especially two or more) should equate to an absolute contraindication. But if there are no other relative contraindications, the age 65-74 has the greatest benefit (NNT = 38 to prevent death from any cause). This effect is even more important for patients age >= 75.
Indeed, there has been much debate over whether ANY benefit exists for patients age >= 75. There was no statistically significant benefit for age >= 75 in the FTT-1994 meta-analysis. Additionally, multiple subsequent large observational studies reported more harm than benefit in this age group. This prompted many to conclude that age >= 75 should be an absolute contraindication to fibrinolytic treatment of STEMI. However, an evidence-based argument eventually emerged which argued that RCT's were indicated to definitively answer the question but that in the interim there exists enough data to infer benefit in this age group. The evidence consists mainly of two findings: 1) a re-analysis of the FTT-1994 outcomes with modern inclusion criteria (mainly symptoms < 12 hours and only ST-elevation/LBBB for diagnostic criteria) showed a benefit in this age group (OR 0.84, 95% CI 0.72 to 0.98) and 2) the GUSTO-1 trial directly compared TPA to streptokinase and showed a mortality benefit in the age >=75 group. Considering that many of the FTT-1994 patients received streptokinase, this is reasonably strong indirect evidence to support tPA in the age >= 75 group.[18] However, authors continue to emphasize that RCT's are indicated to definitively answer this question because a small amount of bias in this data could lead to embracing a treatment which produces substantial harm (i.e. with regards to death).[19] In summary, fibrinolytics are probably beneficial for age >= 75, but clinicians should be extremely vigil for the presence of other relative contraindications and should not be surprised if new RCT's eventually reverse this conclusion.
The chart below illustrates the NNT's by age group (to prevent one deaths) of the original FTT (1994) estimates versus those of the Estess (2002) re-analysis of the same data. Of note, there are three reasons to believe that even the Estess (2002) results are under-estimates of the benefit (all-cause mortality reduction) of modern practice:
- Many of the patients in the FTT dataset received streptokinase (which has subsequently been shown to be less effective).
- Fibrinolytics are now dosed by weight (which decreases the rate of intracranial hemorrhage in lower weight persons).
- Heparin/Lovenox and platelet inhibitor dosing is now adjusted for age and/or renal function (which also lowers the rate of intracranial hemorrhage).
Fibrinolytic Consent Data-Sources
- When administered within 6 hours of pain onset, about 1 in 40 persons will have their life saved.
- When administered between 6-12 hours after pain onset, about 1 in 60 persons will have their life saved.
- About 1 in 100 persons will experience non-life-threatening bleeding
- The data for these statements was taken directly from TheNNT.com (archived 09/24/2020) which quotes FTT-1994.[15]
- About 1 in 100-250 persons will experience bleeding into the brain which usually results in either death or significant disability.
- This statement synthesizes the ICH risk from the data in the ICH table above (for persons below age 75).
Fibrinolytic Checklist Sources
- Table 6. Contraindications and Cautions for Fibrinolytic Therapy in STEMI. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction (Archived)
- UpToDate.com: Acute ST-elevation myocardial infarction: The use of fibrinolytic therapy (Literature review current through: Oct 2020. | This topic last updated: Apr 21, 2020.)
- UpToDate.com: Absolute and relative contraindications to the use of thrombolytic therapy in patients with acute ST-elevation myocardial infarction (Graphic 68784 Version 11.0)
- The relative risks specified for intracranial hemorrhage in the checklist are from Gurwitz-1998.[16]
- BP > 180/110 as an ABSOLUTE contraindication in patients at LOW risk of cardiac death but a RELATIVE contraindication in patients at a HIGH risk of cardiac death.
- Patients with a lower risk of death from STEMI have a lower likelihood of benefit from fibrinolysis; conversely, patients at higher risk of death from STEMI have a higher likelihood of benefit from fibrinolysis. The increased risk of ICH with BP > 180/110 still yields a net benefit in those at high risk of death from STEMI but not in those at low risk of death from STEMI. Alyward et al.[20] discusses the outcomes associated with elevated BP at presentation versus risk of cardiac death, and Estess et al.[18] discusses risk of death and likelihood of benefit based upon age in the context of modern inclusion criteria for fibrinolytic treatment of STEMI. But be warned: this rabbit-hole is deep, dark, and yields only soft conclusions and 20-year-old pleas for future research (which is unlikely to occur at this point because PCI has become the standard of care in the types of institutions which perform randomized trials).
Credits
Lead Author[s]
- Andrew W. Swartz, MD
Co-author[s]
- Megan Young, DO
Reviewer[s]
- Ann Marie Garritano, MD
References
- ↑ 1.0 1.1 Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60(16):1581-1598. doi:10.1016/j.jacc.2012.08.001
- ↑ Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139-e228. doi:10.1016/j.jacc.2014.09.017
- ↑ Roffi M, Patrono C, Collet J-P, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(3):267-315. doi:10.1093/eurheartj/ehv320
- ↑ 4.0 4.1 4.2 Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018). Journal of the American College of Cardiology. 2018;72(18):2231-2264. doi:10.1016/j.jacc.2018.08.1038
- ↑ Berwanger O, Santucci EV, de Barros E Silva PGM, et al. Effect of Loading Dose of Atorvastatin Prior to Planned Percutaneous Coronary Intervention on Major Adverse Cardiovascular Events in Acute Coronary Syndrome: The SECURE-PCI Randomized Clinical Trial. JAMA. 2018;319(13):1331-1340. doi:10.1001/jama.2018.2444
- ↑ 6.0 6.1 Nicholls SJ, Psaltis PJ. Lipid Lowering in Acute Coronary Syndrome: Is Treatment Early Enough? JAMA. 2018;319(13):1325-1326. doi:10.1001/jama.2018.2426
- ↑ Vale N, Nordmann AJ, Schwartz GG, et al. Statins for acute coronary syndrome. Cochrane Database of Systematic Reviews. 2014;(9). doi:10.1002/14651858.CD006870.pub3
- ↑ Patel V, Runde D. Statins for Acute Coronary Syndrome. TheNNT. Accessed October 3, 2020. https://web.archive.org/web/20201003231224/https://www.thennt.com/nnt/statins-for-acute-coronary-syndrome/
- ↑ STrategic Reperfusion in Elderly Patients Early After Myocardial Infarction - ClinicalTrials.gov. Accessed November 12, 2020. https://clinicaltrials.gov/ct2/show/NCT02777580
- ↑ Armstrong PW, Bogaerts K, Welsh R, et al. The Second Strategic Reperfusion Early After Myocardial Infarction (STREAM-2) study optimizing pharmacoinvasive reperfusion strategy in older ST-elevation myocardial infarction patients. Am Heart J. 2020;226:140-146. PMID:32553932. doi:10.1016/j.ahj.2020.04.029
- ↑ Andrade-Castellanos CA, Colunga-Lozano LE, Delgado-Figueroa N, Magee K. Heparin versus placebo for non-ST elevation acute coronary syndromes. Cochrane Database Syst Rev. 2014;(6):CD003462. doi:10.1002/14651858.CD003462.pub3
- ↑ Group GW and TN. Heparin for Acute Coronary Syndromes. TheNNT. https://www.thennt.com/nnt/heparin-for-acute-coronary-syndromes/. Accessed February 2, 2020. Archived
- ↑ Helman A. Heparin for ACS and STEMI | Journal Jam Podcast. Emergency Medicine Cases. January 2020. https://emergencymedicinecases.com/journal-jam-heparin-acs-stemi/. Accessed February 2, 2020. (Archived)
- ↑ No More Heparin for NSTEMI? REBEL EM - Emergency Medicine Blog. February 2019. https://rebelem.com/no-more-heparin-for-nstemi/. Accessed February 2, 2020. (Archived)
- ↑ 15.0 15.1 15.2 15.3 Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Lancet. 1994;343(8893):311-322. DOI:10.1016/S0140-6736(94)91161-4
- ↑ 16.0 16.1 16.2 Gurwitz JH, Gore JM, Goldberg RJ, et al. Risk for intracranial hemorrhage after tissue plasminogen activator treatment for acute myocardial infarction. Participants in the National Registry of Myocardial Infarction 2. Ann Intern Med. 1998;129(8):597-604. doi:10.7326/0003-4819-129-8-199810150-00002
- ↑ 17.0 17.1 Brass LM, Lichtman JH, Wang Y, Gurwitz JH, Radford MJ, Krumholz HM. Intracranial hemorrhage associated with thrombolytic therapy for elderly patients with acute myocardial infarction: results from the Cooperative Cardiovascular Project. Stroke. 2000;31(8):1802-1811. doi:10.1161/01.str.31.8.1802
- ↑ 18.0 18.1 Estess JM, Topol EJ. Fibrinolytic treatment for elderly patients with acute myocardial infarction. Heart. 2002;87(4):308-311. PMID: 11906993. doi:[[ https://doi.org/10.1136/heart.87.4.308%7C10.1136/heart.87.4.308]]
- ↑ Van de Werf F. Reperfusion treatment in acute myocardial infarction in elderly patients. Kardiol Pol. 2018;76(5):830-837. PMID: 29633231. doi:10.5603/KP.a2018.0092
- ↑ Aylward PE, Wilcox RG, Horgan JH, et al. Relation of increased arterial blood pressure to mortality and stroke in the context of contemporary thrombolytic therapy for acute myocardial infarction. A randomized trial. GUSTO-I Investigators. Ann Intern Med. 1996;125(11):891-900. doi:10.7326/0003-4819-125-11-199612010-00004