Phenobarbital for Alcohol Withdrawal
Phenobarbital (PB) is a non-competitive gamma-Aminobutyric acid (GABA) agonist which is an equally effective and safe alternative to benzodiazepines (BZD) for the treatment of alcohol withdrawal syndrome (AWS).[1] Though its use for AWS has waned and consequently many clinicians are now unfamiliar with this regimen, PB has both mechanistic and pharmacokinetic properties which make it more suitable for outpatient monotherapy than BZD.
IV/IM titrated PB is the first-line outpatient medication used for treatment of AWS at the Yukon-Kuskokwim Delta Regional Hospital (YKDRH). Its use is favored because substantial experience at this institution has shown that it is safe, it is effective, it minimizes return visits, and it eliminates the need to dispense abuse-prone medications (i.e. BZD) to abuse-prone patients while in the midst of a substance abuse crisis. Indeed, minimizing high-risk dispensing of abuse-prone medication is important for improving the health of our community.
The treatment of AWS with phenobarbital (or barbiturates [BBT] in general) is not new, but it has fallen so out of favor that many clinicians are unfamiliar with this use. However, there is ample published evidence, both old and new, indicating that phenobarbital is at least equally safe and effective compared with benzodiazepines.
This page focuses upon the use and pharmacology of phenobarbital. For more general information about AWS and local practices, see Alcohol Withdrawal in the YK Delta.
Treatment Principles and Pearls
Sub-sedative dosing
- Alcohol withdrawal is a hyper-alert and/or hyper-autonomic state. The goal of outpatient treatment of alcohol withdrawal is normalization of alertness (i.e. level of consciousness) and autonomic function, not sedation. If a sedative level of [any] medication is required for AWS symptom control, then admission is often indicated.
Dose titration
- Patients’ individual medication requirements are unpredictable. Therefore no standard PB dose is expected to be effective for all patients. Rather, an initial IV (or IM) PB dose of 260 mg is given, and then every 30 minutes (or 60 minutes for IM) an additional 130mg are given until the desired effect is achieved. Using this regimen, many patients will require repeat doses, but none should end up “sedated.”
- The standard 260mg/130mg regimen works well for average size patients. This regimen yields weight-based doses of 3.7 mg/kg and 1.9 mg/kg in a 70 kg patient. However, for patients substantially below or above 70 kg, clinicians should consider administering 4 mg/kg with subsequent doses of 2 mg/kg. For convenience, these doses can be rounded to the nearest 130 mg increment (i.e. the amount in a single vial). For example, weight-based doing for a 125 kg patient would yield 500 mg and 250 mg doses, and these can be safely rounded to 520mg and 260mg for ease of administration. Weight-based dosing prevents sedating unusually small patients while preventing excessively long visits for unusually large patients. Dosing in whole-vial increments minimizes the risk of dosing errors and eliminates the effort required to precisely dose from a 1mL vial.
Dosing interval
- In pharmacokinetic studies of rapid PB boluses, on average, PB reaches 90% of peak brain concentration by 19 minutes, 95% by 22 minutes, and 100% by 30 minutes.[2] Yet clinical judgment must still be used. At 30 minutes after a PB dose, if the patient has “almost” complete symptom relief, the clinician should appreciate the possibility of a small amount of biological variability and consider reevaluating after another 10 minutes, as it is possible that a small additional effect may become apparent. But if the dose is clearly inadequate at 30 minutes, further waiting is very unlikely to reveal any substantial further effect.
Sleep after symptom control
- When patients’ hyper-alert/hyper-autonomic state is normalized, many will lightly nap. But such patients easily awaken to voice or light touch and they easily meet all discharge criteria. Rather than being “sedated,” such patients are merely exhibiting the normal physiologic response in the absence of discomfort to a 24-48 hour sleep deficit (which is the norm for patients in this situation). Any concern for sedation can usually be answered by asking oneself if the patient’s post-treatment behavior would be considered abnormal for a patient in the ED with a normal mental status, with a substantial sleep deficit, whose discomfort had been relieved. If the answer is “no”, then the patient is not “sedated,” but rather has been returned to a normal level of alertness.
Transient post-infusion effects
- Dizziness/nystagmus/ataxia
- This is a common, transient effect of IV PB administration. Both experience and published reports reveal that this resolves spontaneously in 15-30 minutes. Given PB's exceptionally long half-life, this seems to be attributable to the rapidity of the drug level increase rather than the level itself. After resolution, this is not a contraindication for more PB, but further doses should be infused much more slowly (such as over 30-45 minutes). Importantly, this is not an allergic reaction nor a reaction which warrants a chart alert. This reaction can be thought of as similar to the "red man" effect of vancomycin: it merely requires adjustment of the administration speed and possibly the dosing interval. This effect occurs more frequently when infusing larger doses (such as 390mg or 520mg) over 10 minutes; such larger doses (such as in a very large patient or one with a well-established dose requirement) should be infused over 30-45 minutes.
- Asymptomatic hypotension
- One study (Young et al.[3]) reported that 1 of 62 patients treated with IV PB monotherapy "had an asymptomatic blood pressure fall to 90/60 mm Hg, which rose without intervention within 30 minutes."
Post-treatment tiredness
- Once symptom control has been achieved with PB, most patients complain of feeling "tired". It is unclear if this is an effect of phenobarbital versus an appropriate sensation given their physiologic circumstance. Regardless of etiology, tiredness seems a good indicator that they will be able to sleep.
Evidence of Effectiveness and Safety
Systematic Review / Meta-analysis
- A 2016 systematic review by Mo et al. in the Journal of Critical Care[1] concluded that "barbiturates alone or in combination with BZDs are at least as effective as BZDs in the treatment of AWS. Furthermore, barbiturates appear to have acceptable tolerability and safety profiles, which were similar to those of BZDs in patients with AWS." This review included three randomized controlled trials (RCT's) and four observational studies. Importantly, the authors observed that "none of these studies demonstrated inferiority of barbiturates to BZDs in the management of AWS." While this review firmly establishes PB's similar pharmacological effectiveness compared to BZD's, only one of the included studies (an RCT by Hendey et al.[4]) focused solely upon outpatient treatment. Though its sample size was small (25 in PB group, 19 in BZD group), Hendey et al. reported statistically nonsignificant trends toward better outcomes in the PB group.
- Other systematic reviews[5][6] have only indirectly assessed the effectiveness of PB for AWS. These reviews have grouped all studies which compared any anti-convulsant to a BZD, and then concluded that anti-convulsants (as a group) are inferior to BZDs for treatment of AWS. This grouping of PB with other anti-convulsants badly biases the results against PB and does not seem justified. The mechanism of action of both PB and BZDs is non-competitive agonism of the GABAA ion channel and both of these medication have been long known to display cross-tolerance with each other and alcohol. Conversely, the other anti-convulsants work via different mechanisms. Therefore grouping PB with the other anti-convulsants is inappropriate and seems to merely reflect how the authors think about the drugs rather than their pharmacological properties.
Studies of ED/Outpatient Treatment
- Young et al. (1987, prospective uncontrolled study, n=62)[3]
- 62 patients received IV PB loading dose in the emergency department. The protocol was an initial 260mg which was followed by 130mg increments, but the dosing interval is unclear. The mean loading dose was 598 mg (8.4 mg/kg) and the subsequent mean serum PB level was 13.9 mcg/mL. Four patients experienced minor, self-limited adverse reactions (asymptomatic hypotension, ataxia, or lethargy) which resolved without intervention. All patients were discharged from the ED and none returned for further care during the following week.
- Hendey et al. (2011, RCT of PB vs lorazepam, n=25/19)[4]
- 44 ED patients were randomized (25 to IV-PB, 19 to LZ). PB patients received a 230 mg initial dose followed by 130mg subsequent doses (dosing interval was "at the discretion of the treating physician"). Mean PB dose was 509 mg (range 260-910 mg); mean number of doses was 2.9 (range 1-6). There were no outcome differences at 48 hours, and importantly, there was no significant difference in length of ED stay (267 min for PB versus 256 min for LZ). Fewer PB patients required admission (12 vs. 16). The authors concluded that "phenobarbital and LZ were similarly effective in the treatment of mild/moderate alcohol withdrawal in the ED and at 48 hours."
- Nelson et al. (2019, retrospective cohort study)[7]
- 300 ED patients: 100 received IV-PB alone, 100 received IV-PB + lorazepam, and 100 received diazepam alone. Initial PB dose was 260mg and subsequent doses were 130mg (dosing interval is not specified). Authors' conclusions: "Incorporating phenobarbital into a benzodiazepine based protocol or as sole agent led to similar rates of ICU admission, length of stay, and need for mechanical ventilation in patients treated for alcohol withdrawal in the emergency department."
Historical Reports
- PB has long been used for treatment of delirium tremens (DT). Though it has fallen out of favor in most places, a minority of countries and institutions have continued to use it as first line treatment of AWS.
- A 2010 study by Michaelsen in the Danish Medical Bulletin[8] states BBTs have been used for treatment of DT in Denmark "for over 100 years."
- A 2006 RCT of gabapentin for AWS[9] compared it to a control group which received PB. The authors gave the following explanation in the methods section:
- Those randomized to the experimental group received a protocol using gabapentin, while those in the control group received a phenobarbital protocol that is customary in our inpatient detoxification service. Phenobarbital was used in this study rather than benzodiazepines because it is the detoxification medication that the clinical staff is most familiar with.
- A 1995 United States nationwide survey of inpatient alcohol treatment centers[10] reported that approximately 10% primarily used PB for treatment of AWS.
EVIDENCE SUMMARY
- In landmark studies by Kaim and Klett in 1969[11] and 1972,[12] both BBTs and BZDs were shown to be equally effective in treatment of AWS. Both of these drug classes had been previously known to exhibit cross dependance with each other as well as alcohol, and the subsequent discovery that these structurally unrelated compounds are both GABA agonists was critical to understanding the pathophysiology of AWS.
- Though BZDs have become the standard of care, this seems mostly attributable to a larger movement away from inpatient treatment and toward outpatient treatment with oral medications. Dispensing BZDs to patients is clearly safer than dispensing PB/BBTs. Yet when the dose is titrated to effect by a physician and patients are not dispensed medication, there is ample evidence that PB is equally safe and effective compared to BZD.
- Numerous articles advise caution with regard to PB by alluding to concerns about respiratory depression, but no evidence of adverse effects is ever presented or cited. Therefore these concerns seem to originate more from the authors' lack of familiarity with the use of IV-PB in this setting than from documented outcomes. However, it must be pointed out that PB should never be dispensed to a patient for symptom-triggered dosing, as this would present an extreme risk of unintentional lethal overdose. PB use for AWS must be limited to titrating the dose to the desired effect, and then discharging the patient without additional medication. There have been no reports of iatrogenic overdoses using this protocol, and the drug packaging is a strong mitigating factor to prevent drug errors: At YKDRH, PB is only available in 130mg vials. Therefore the initial dose is two vials and the subsequent doses are one vial. A dangerous overdose (i.e. one yielding respiratory depression) would require accidentally drawing up 20-30 vials, which would be very labor intensive and very likely produce questions prior to administration. (PB is also marketed in 65mg vials, but that would require even more vials to produce an overdose.)
Regulatory Information and Packaging
- PB is not approved by the U.S. FDA; this is likely due its use preceding the creation of the FDA (in 1938).
- DEA Schedule: IV
- Manufacturer's package insert (electronic, printable, archived printable)[13].
- How Supplied:
- Phenobarbital Sodium Injection, 65 mg/mL, 1 mL vials packaged in 25s (NDC 0641-0476-25)
- Phenobarbital Sodium Injection, 130 mg/mL, 1 mL vials packaged in 25s (NDC 0641-0477-25)
- Phenobarbital Sodium Injection, 65 mg/mL, 1 mL vials packaged in 25s (NDC 0641-0476-25)
Pharmacology
The following are some of the important properties of PB with regard to outpatient treatment of AWS.
Classification
- Chemical Class
- PB belongs to the chemical class barbiturate.
- Clinical Class
- Like all barbiturates, PB is a sedative/hypnotic.[14] However, it is almost never used for this purpose.
- PB is also classified as an anti-convulsant.
- In anesthetic doses, all BBTs have an anti-convulsant effect. However, PB is the only BBT which has an anti-convulsant effect at sub-sedative doses. (Historically, two closely related derivatives [mephobarbital and metharbital] also had an anti-convulsant effect at sub-sedative doses).[14]
Mechanism of Action
- GABA agonist
- The binding of GABA to its receptor inhibits nerve depolarization. Like all barbiturates, PB's sedative/hypnotic effect occurs primarily via noncompetitive agonism of the GABAA receptor on the GABA-mediated ion channel. Like BZDs, PB/BBTs do not themselves open the channel and thus GABA is still required. PB/BBTs bind at a different site on the GABAA receptor than BZDs.[15]
- When PB/BBTs bind to the GABAA receptor, three effects occur:
- Both 2 and 3 indicate a potential additive (or even synergistic) effect when co-administered with BZDs in severe withdrawal. This is consistent with statistically nonsignificant trends in published studies.[1]
- Glutamate antagonist
- The binding of glutamate to its receptor stimulates nerve depolarization. At sub-sedative doses PB/BBTs also inhibit the AMPA subtype of glutamate receptors; however, PB/BBT do not effect the NMDA subtype.[15] This glutamate antagonism is an anti-convulsive effect which is separate from PB's GABAergic effect.[16]
Distribution
Venous concentration versus infused dose
| Group | PB Dose (mg/kg) |
Serum Concentration (μ/mL) |
Conc : Dose Ratio |
|---|---|---|---|
| Tremulous (n=48) | 8.5 | 14.0 | 1.65 |
| Seizures (n=38) | 8.3 | 13.8 | 1.66 |
| Alcoholic liver disease (n=21) | 8.2 | 13.7 | 1.67 |
| ALL (n=62) | 8.4 | 13.9 | 1.65 |
- Young (1987)[3] compared infused PB doses to post-infusion serum PB concentrations in adults presenting to the ED for AWS, and reported that the serum PB level rose 1.65 μg/mL for each mg/kg of PB infused. Though no standard deviation is reported, the similarity of outcomes in different subgroups (including those with alcoholic liver disease) is indicative of very minimal variability of the final serum concentration versus the infused dose (see Table-1).
Venous concentration versus time
- Following rapid bolus injection in adults, PB quickly distributes in the blood, achieving peak venous concentration at about 3 minutes and settling into a relatively steady concentration by 5 minutes.[17]
Brain concentration versus time
- Paulson et al. showed that after rapid bolus injection, on average, PB reaches 90% of peak brain concentration by 19 minutes, 95% by 22 minutes, and 100% by 30 minutes.[2]
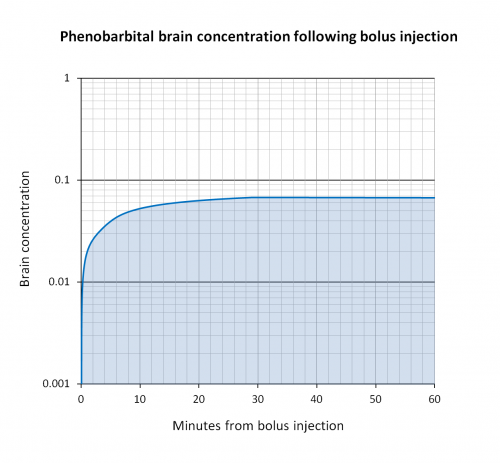
- Alternately, the manufacturer package insert (updated 12-10-2018) states that peak brain concentration is reached within 15 minutes of IV administration.[13]
Bioavailability
- Intramuscular bioavailability is 75-80% (based upon a 1978 study of five young adult males[18]).
Dosing
Infused dose, Venous concentration, and Clinical Effect
- Figure-2 is a nomagram illustrating the expected serum PB levels based on infusion dose (in mg/kg) and it also shows expected effects at particular concentrations as well as the dose ranges reported in applicable studies.
- Figure-2 is a nomagram illustrating the expected serum PB levels based on infusion dose (in mg/kg) and it also shows expected effects at particular concentrations as well as the dose ranges reported in applicable studies.
Recommended Dosing
- Intravenous
- Initial: 260mg (or approximately 4 mg/kg) over 10 minutes
- Subsequent: 130mg (or approximately 2 mg/kg) over 10 minutes, repeat every 30 minutes until adequate symptom relief
- Infusion Rate: ≤ 60 mg/min (adults)[13]
- Intramuscular
- Initial: 260mg (or approximately 4 mg/kg)
- Subsequent: 130mg (or approximately 2 mg/kg) every 60-90 minutes until adequate symptom relief
- Dosing Pearls
- Weight-based dosing should be considered for patients who are particularly under or overweight.
- It is convenient to save two orders (i.e. one for 260mg and one for 130mg) which specify administration "over 10 minutes via infusion pump." This provides standardization, safety, and efficient use of nursing time.
- Patients must never be discharge with a supply of oral PB for symptom-triggered dosing due to an extreme risk of unintended lethal overdose.
- If adequate symptom relief cannot be obtained with a sub-sedative dose of PB, then the patient should be admitted for dual-treatment with a BZD. These patients warrant consideration for transfer for ICU care.
- Oral Dosing
- Oral dosing information is for hypothetical purposes only. There is no standard indication for this regimen at YKDRH, but in highly unusual circumstances this information might be useful.
- Oral PB has 95-100% bioavailability.
- Patients must never be discharge with a supply of oral PB for symptom-triggered dosing due to an extreme risk of unintended lethal overdose.
- Three uncontrolled observational studies have reported results of an orally titrated PB loading regimen for treatment of "sedative/hypnotic" withdrawal.[19][20][21] The three studies involved a total of 87 patients, and they administered PB 120mg orally each hour until the desired therapeutic affect was achieved. There were zero occurrences of adverse effects or over-sedation despite minimal monitoring (nursing contact once per hour). The average final doses were 1440mg, 1315mg, and 1180, respectively. The authors concluded the regimen is safe, effective, and efficient (i.e. requires minimal nursing resources). The caveat to consider is that these patients were withdrawing from barbiturates, therefore they had developed tolerance. If used for alcohol withdrawal, lower final doses should be anticipated.
Elimination
- The half-life of PB is frequently reported as 100 hours or as 80-120 hours.[22] This is an accurate generalization, but the generalization hides the variation among different populations.
- Non-barbiturate-habituated adults: approximately 80-85 hours.
- Barbiturate-habituated adults: approximately 55-60 hours.
- 25-50% of PB is excreted unchanged in the urine. Alkalinization of the urine enhances elimination. Hepatic metabolism produces only inactive metabolites which are excreted in the urine and feces.
- Renal insufficiency has little effect upon elimination half-life, but severe renal failure likely prolongs elimination.[23]
- Hepatic cirrhosis has been shown to prolong elimination whereas acute viral hepatitis does not.[24]
Adverse Effects
- Common
- Transient post-infusion dizziness, ataxia, and/or nystagmus
- Injection-site reactions
- Uncommon
- Allergic reactions
- Very uncommon
- Exfoliative dermatitis, Stevens-Johnson syndrome, toxic epidermic necrolysis
- With overdose
- Coma
- Respiratory depression
- Vomiting
- With severe overdose
- Apnea
- Cardiovascular collapse
- Cardiac conduction
- Neither the manufacturer package insert nor Lexicomp report cardiac conduction effects (such as QT prolongation) or increased risk of arrhythmias.
Cautions
- Acute or chronic pain
- Airway obstruction
- Respiratory distress
- Significant blood level of alcohol or other sedatives
Contraindications
- History of porphyria
Pregnancy
- Pregnancy Risk Factor
- Injection: Category D
- PB readily crosses placenta and yields fetal blood levels similar to maternal levels. Data from retrospective, case-controlled studies suggest an association with increased fetal abnormalities and malformations; however, this data has risk of confounding from the seizure disorder and other co-prescribed seizure medications. The manufacturer recommends that Phenobarbital should be used during pregnancy only when clearly indicated.[13] During labor, PB does not effect uterine contractions, but it does cause newborn respiratory depression.[25]
Lactation
- PB readily passes into breast milk. The major effect is infant drowsiness but there are several reports of severe infant sedation. PB does not effect milk production.
- See TOXNET/LACTMED: PHENOBARBITAL for details.
Toxicology
- PB blood levels above 80 μg/mL are considered "potentially" lethal, whereas lethal overdose is usually associated with levels of 100-200 μg/mL.[13] For a 70 kg person, these levels would result from ingestions of 3,394mg, 4,242mg, and 8,485mg, respectively. Six to ten grams is a commonly cited lethal ingestion dose range.[26][27]
- Overdose management is generally supportive: airway maintenance/protection, mechanical ventilation, volume and vasopressors as needed for circulatory support.
- The is no PB/BBT antidote. Due to the extremely long half-life of PB, hemodialysis is often used to rapidly decrease serum levels.[27]
Abbreviations
- AWS: Alcohol Withdrawal Syndrome
- BBT: Barbiturate[s]
- BZD: Benzodiazepine[s]
- GABA: Gamma-aminobutyric acid
- PB: Phenobarbital
- YKDRH: Yukon-Kuskokwim Delta Regional Hospital
Related Topics
Alcohol Withdrawal in the YK Delta
External Links
PubMed (all settings):
- "alcohol withdrawal"[Title/Abstract] AND (phenobarb*[Title/Abstract] OR barbiturat*[Title/Abstract])
PubMed (ED/outpatient):
Manufacturer's package insert (electronic, printable, archived printable)[13].
Lexicomp/Lexi-Drugs: Phenobarbital
Lexicomp/Lexi-Tox: Phenobarbital
Contributors
Authors
- Andrew W. Swartz, MD
Reviewers:
- Megan Young, MD
- Travis Nelson, MD
- Tara Lathrop, MD (Emergency Department Service Chief)
References
- ↑ 1.0 1.1 1.2 Mo Y, Thomas MC, Karras GE. Barbiturates for the treatment of alcohol withdrawal syndrome: A systematic review of clinical trials. J Crit Care. 2016;32:101-107. doi:10.1016/j.jcrc.2015.11.022
- ↑ 2.0 2.1 2.2 Paulson OB, Györy A, Hertz MM. Blood-brain barrier transfer and cerebral uptake of antiepileptic drugs. Clin Pharmacol Ther. 1982;32(4):466-477. doi:10.1038/clpt.1982.190
- ↑ 3.0 3.1 3.2 3.3 Young GP, Rores C, Murphy C, Dailey RH. Intravenous phenobarbital for alcohol withdrawal and convulsions. Ann Emerg Med. 1987;16(8):847-850. doi:10.1016/s0196-0644(87)80520-6
- ↑ 4.0 4.1 Hendey GW, Dery RA, Barnes RL, Snowden B, Mentler P. A prospective, randomized, trial of phenobarbital versus benzodiazepines for acute alcohol withdrawal. Am J Emerg Med. 2011;29(4):382-385. doi:10.1016/j.ajem.2009.10.010
- ↑ Minozzi S, Amato L, Vecchi S, Davoli M. Anticonvulsants for alcohol withdrawal. Cochrane Database Syst Rev. 2010;(3):CD005064. doi:10.1002/14651858.CD005064.pub3
- ↑ Amato L, Minozzi S, Davoli M. Efficacy and safety of pharmacological interventions for the treatment of the Alcohol Withdrawal Syndrome. Cochrane Database Syst Rev. 2011;(6):CD008537. doi:10.1002/14651858.CD008537.pub2
- ↑ Nelson AC, Kehoe J, Sankoff J, Mintzer D, Taub J, Kaucher KA. Benzodiazepines vs barbiturates for alcohol withdrawal: Analysis of 3 different treatment protocols. Am J Emerg Med. 2019;37(4):733-736. doi:10.1016/j.ajem.2019.01.002
- ↑ Michaelsen IH, Anderson JE, Fink-Jensen A, Allerup P, Ulrichsen J. Phenobarbital versus diazepam for delirium tremens--a retrospective study. Dan Med Bull. 2010;57(8):A4169.
- ↑ Mariani JJ, Rosenthal RN, Tross S, Singh P, Anand OP. A randomized, open-label, controlled trial of gabapentin and phenobarbital in the treatment of alcohol withdrawal. Am J Addict. 2006;15(1):76-84. doi:10.1080/10550490500419110
- ↑ Saitz R, Friedman LS, Mayo-Smith MF. Alcohol withdrawal: a nationwide survey of inpatient treatment practices. J Gen Intern Med. 1995;10(9):479-487. doi:10.1007/bf02602395
- ↑ Kaim SC, Klett CJ, Rothfeld B. Treatment of the acute alcohol withdrawal state: a comparison of four drugs. Am J Psychiatry. 1969;125(12):1640-1646. doi:10.1176/ajp.125.12.1640
- ↑ Kaim SC, Klett CJ. Treatment of delirium tremens. A comparative evaluation of four drugs. Q J Stud Alcohol. 1972;33(4):1065-1072.
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 Phenobarbital sodium [package insert]. Eatontown, NJ: West-Ward Pharmaceuticals Corporation; 2018.
- ↑ 14.0 14.1 Harvey SC. Hypnotics and Sedatives: The Barbiturates. In: Goodman LS, Gilman A, eds. The Pharmacologic Basis of Therapeutics. 5th ed. New York: MacMillan Publishing Co., Inc.; 1975:124-136.
- ↑ 15.0 15.1 15.2 15.3 15.4 Hobbs WR, Rall TW, Verdoorn TA. Ch 17: Hypnotics and Sedatives; Ethanol. In: Hardman JG, Limbird LE, eds. The Pharmacological Basis of Therapeutics. 9th ed. New York: McGraw-Hill; 1996:361-398.
- ↑ Nardou R, Yamamoto S, Bhar A, Burnashev N, Ben-Ari Y, Khalilov I. Phenobarbital but Not Diazepam Reduces AMPA/kainate Receptor Mediated Currents and Exerts Opposite Actions on Initial Seizures in the Neonatal Rat Hippocampus. Front Cell Neurosci. 2011;5:16. doi:10.3389/fncel.2011.00016
- ↑ Bøjholm S, Paulson OB, Flachs H. Arterial and venous concentrations of phenobarbital, phenytoin, clonazepam, and diazepam after rapid intravenous injections. Clin Pharmacol Ther. 1982;32(4):478-483. doi:10.1038/clpt.1982.191
- ↑ Viswanathan CT, Booker HE, Welling PG. Bioavailability of oral and intramuscular phenobarbital. J Clin Pharmacol. 1978;18(2-3):100-105. PMID:624773
- ↑ Robinson GM, Sellers EM, Janecek E. Barbiturate and hypnosedative withdrawal by a multiple oral phenobarbital loading dose technique. Clin Pharmacol Ther. 1981;30(1):71-76. doi:10.1038/clpt.1981.129
- ↑ Janecek E, Kapur BM, Devenyi P. Oral phenobarbital loading: a safe method of barbiturate and nonbarbiturate hypnosedative withdrawal. CMAJ. 1987;137(5):410-412. PMID: 3621099/
- ↑ 1. Loder E, Biondi D. Oral phenobarbital loading: a safe and effective method of withdrawing patients with headache from butalbital compounds. Headache. 2003;43(8):904-909. doi:10.1046/j.1526-4610.2003.03171.x
- ↑ Martin PR, Bhushan CM, Kapur BM, Whiteside EA, Sellers EM. Intravenous phenobarbital therapy in barbiturate and other hypnosedative withdrawal reactions: a kinetic approach. Clin Pharmacol Ther. 1979;26(2):256-264. doi:10.1002/cpt1979262256
- ↑ Asconapé JJ. Use of antiepileptic drugs in hepatic and renal disease. Handb Clin Neurol. 2014;119:417-432. doi:10.1016/B978-0-7020-4086-3.00027-8
- ↑ Kutt H, Winters W, Scherman R, Mcdowell F. Diphenylhydantoin and Phenobarbital Toxicity. The Role of Liver Disease. Arch Neurol. 1964;11:649-656. doi:10.1001/archneur.1964.00460240081011
- ↑ Lexicomp Online, Lexi-Drugs, Hudson, Ohio: Wolters Kluwer Clinical Drug Information, Inc.; 2019; Sept. 17, 2019.
- ↑ Lindberg MC, Cunningham A, Lindberg NH. Acute phenobarbital intoxication. South Med J. 1992;85(8):803-807. doi:10.1097/00007611-199208000-00004
- ↑ 27.0 27.1 Hoyland K, Hoy M, Austin R, Wildman M. Successful use of haemodialysis to treat phenobarbital overdose. BMJ Case Rep. 2013;2013. doi:10.1136/bcr-2013-010011
